Mechanism of Action
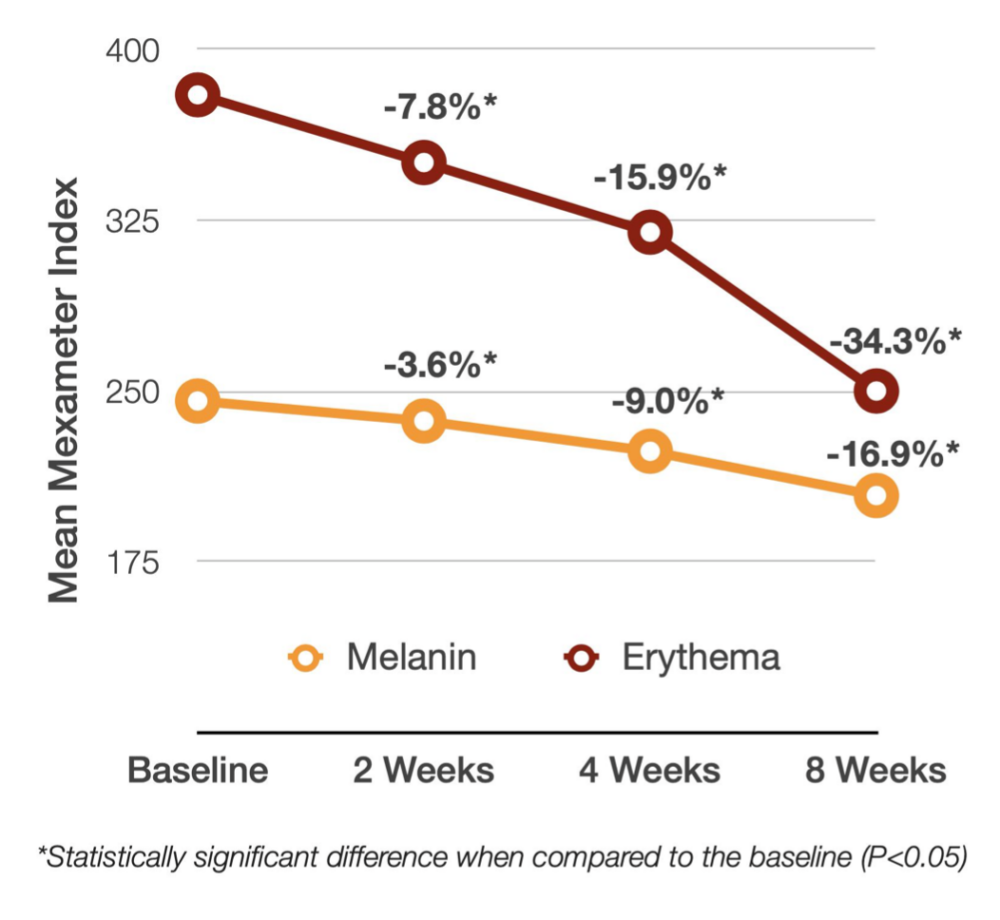
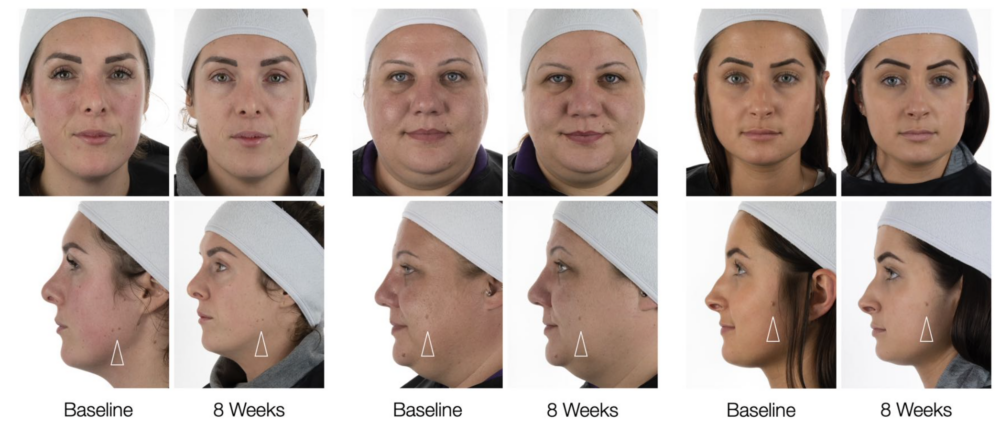
Tranexamic Acid (TXA) is a third-generation bioactive that prevents and soothes both redness and dark spots.
External skin disruptors (UV light, shaving, stripping solvents, detergents, etc.) cause the keratinocytes in the epidermis to produce signal mediators (e.g., plasminogen). These mediators initiate a cascade of events in the skin including inflammation, atypical plasmin activity, proliferation of keratinocytes, desquamation, melanocyte differentiation, increased tyrosinase activity, and transfer of melanosomes to upper layers. The result is unevenly pigmented, dull skin.
TXA inhibits plasmin activity, decelerating the above processes while promoting a more uniform skin tone and faster skin barrier recovery.
Enhancing Skin Penetration
The permeability of TXA through the skin is insufficient due to its hydrophilic nature and strong hydrogen-bonding capacity.
Esters of TXA, like TXVector, offer a higher level of skin-targeting and are broken down by epidermal esterases to the active TXA form.
Formulation Benefits
TXVector is a smart delivery form of TXA that is notably easier to formulate compared to other TXA esters. It also works as the primary emulsifier (o/w).
TXVector can be dissolved and dispersed in water, and remains stable over time without separation or aggregation.
One part of TXVector delivers ≈ 0.33 parts of TXA.